Implementing Electronic Signatures for Sonendo®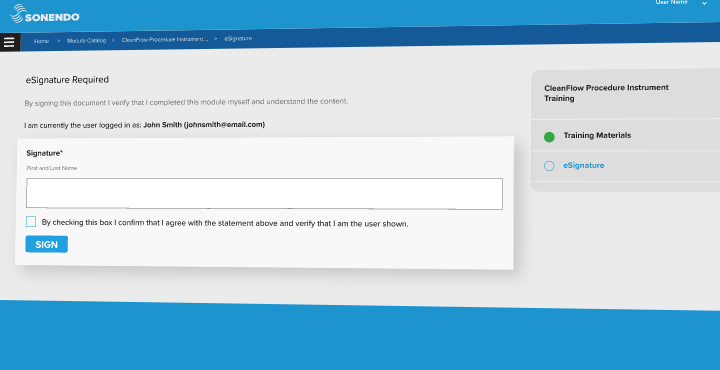
As a medical device manufacturing company, Sonendo® needed a way to ensure authentic and accurate completion of onboarding and continuing education for its practice partners. Konvert™ took on the request and added an e-signature capability to its learning management system (LMS) that allows Sonendo’s users to validate their online learning at the end of a module.
In order to offer this feature, Konvert had to ensure Food and Drug Administration (FDA) compliance with Part 11 of the FDA’s Title 21 by adding three additional features:
Log out inactive users
Once enabled, a user will be automatically logged out if a certain duration of inactivity passes. This duration can be set by the admin.
Force password change
Once enabled, users will be prompted to change their password after a customized duration of time set by the admin.
Audit log
This feature allows admins the opportunity to review when and where actions are taken within the Konvert Portal. Depending on the action, an admin can perform in-depth auditing down to specific LMS modules or actions related to a specific user.
Through the implementation of regulatory features,
Konvert ensures complete security of users within the Portal.
With the addition of FDA-compliant e-signature capabilities, Sonendo is able to authenticate training on its endodontic devices. This is a vital component of onboarding and training of its endodontic customers and is significantly helping streamline the validation process.
Get Started with Konvert™
Looking to implement intuitive online learning with e-signature validation capabilities? Contact us today to get started.